PROVIREX Genome Editing Therapies GmbH
Booth number: C120-07
www.provirex.de
About us
PROVIREX Genome Editing Therapies GmbH, based in Hamburg, is a private biotech company dedicated to the development of advanced therapy medicinal products (ATMPs). The company uses its proprietary genome editing technology to precisely remove integrated viral DNA from infected cells, thereby curing life-threatening viral infections. PROVIREX's GMP-compliant S3** clean rooms in Hamburg, which are registered under GenTSV in Germany and were designed for ATMP production, are unique in Germany and guarantee the highest safety and quality standards.
Address
Luruper Hauptstrasse 1
22547 Hamburg
Germany
E-mail: jan.claasvonjachmann@provirex.de
Phone: +49 40 9999 69190
Internet: www.provirex.de
Products & Services
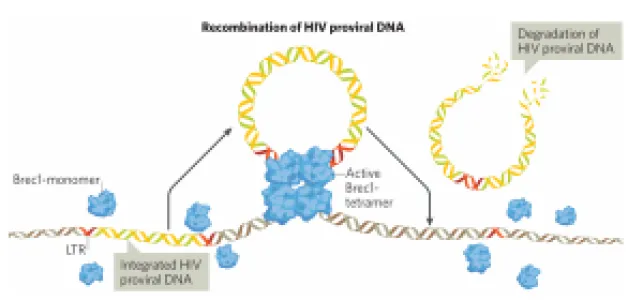
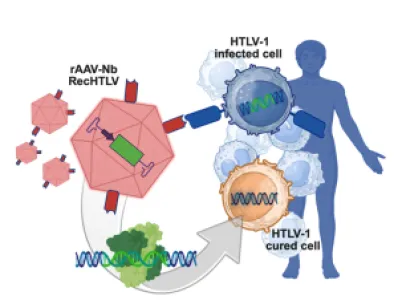
PROVIREX is a company specialising in advanced therapy medicinal products (ATMPs). The company uses its proprietary genome editing technology to precisely remove integrated viral DNA from infected cells, thereby curing life-threatening viral infections. The focus is on therapies to cure HIV infection using a core technology based on highly specific designer recombinases. This technology is currently being tested in a Phase Ib/IIa clinical trial. In addition, platform technologies for cell-specific in vivo delivery of recombinases using AAVs or LNPs are being developed. PROVIREX has established a GMP-compliant Class S3** (Classes D-A) cleanroom, unique in Germany, which has been specifically designed for ATMP production to ensure the highest safety and quality standards. Due to the increased safety classification S3** in accordance with GenTSV in Germany, PROVIREX can expand and transduce potentially HIV-positive cells in these cleanrooms, allowing this infrastructure to be expanded for the production of CAR-T cells for people with HIV and related indications such as lymphomas.